The FDA has a well-intentioned requirement for asymptomatic data.
But that requirement greatly slows down progress on rapid tests, and new data suggests it isn't even necessary anymore!
In light of this new data, the FDA should drop the requirement.
A thread.
But that requirement greatly slows down progress on rapid tests, and new data suggests it isn't even necessary anymore!
In light of this new data, the FDA should drop the requirement.
A thread.

Manufacturers tell us that they are starting with the symptomatic market and will get around to the asymptomatic market "later".
Why?
Because it's *really* hard to get data from positive asymptomatics. You need to screen hundreds of people before you can find even a single case
Why?
Because it's *really* hard to get data from positive asymptomatics. You need to screen hundreds of people before you can find even a single case
The FDA's requirement for asymptomatic data seems reasonable. If a test works in symptomatics, we'd want to know if it also works in asymptomatics. https://www.fda.gov/medical-devices/coronavirus-disease-2019-covid-19-emergency-use-authorizations-medical-devices/vitro-diagnostics-euas
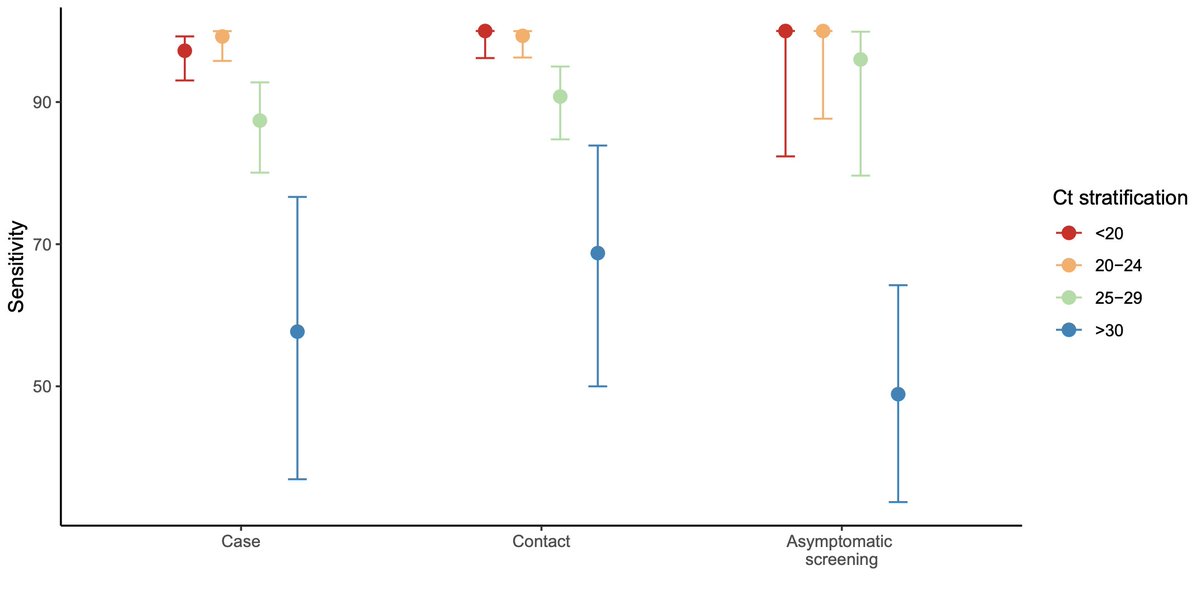
But new data shows that if you break down test results by Ct value – which is what really matters for infectiousness – symptomatic state doesn’t actually matter!
https://www.medrxiv.org/content/10.1101/2020.10.30.20223198v1.full.pdf
https://www.medrxiv.org/content/10.1101/2020.10.30.20223198v1.full.pdf
In other words, if your test has e.g. 90% sensitivity for Ct < 30 in symptomatics, it will almost certainly have approximately 90% sensitivity for Ct < 30 in asymptomatics!
Let's rephrase that, since it's so important:


 If your test has 90% sensitivity for likely infectious symptomatics, it will almost certainly have approximately 90% sensitivity likely infectious asymptomatics!
If your test has 90% sensitivity for likely infectious symptomatics, it will almost certainly have approximately 90% sensitivity likely infectious asymptomatics! 




 If your test has 90% sensitivity for likely infectious symptomatics, it will almost certainly have approximately 90% sensitivity likely infectious asymptomatics!
If your test has 90% sensitivity for likely infectious symptomatics, it will almost certainly have approximately 90% sensitivity likely infectious asymptomatics! 


We see these results in multiple studies on multiple different tests. Not just the preprint linked above, but also in a David Harris study mentioned in a recent NYT article (no pre-print available yet).
For public health purposes, infectiousness is what matters. To get approval, manufacturers should show how sensitivity depends on viral load (or Ct value), without having to conduct slow (and now unnecessary) studies on asymptomatics.
What's at stake is months and months of delays. We need rapid tests now. There's no longer a need to cross our T's and dot our I's for asymptomatics.

 Read on Twitter
Read on Twitter