Finally out! Our work on BRCA1 & BRCA2 have distinct effects on cancer immunity in mice & people. Led by phys-sci superstar @rsamstein, grad-student wonder @chirag_msk, the amazing Xiaoxiao, with tutelage from jedimaster @GeneCollector, & help of many https://www.nature.com/articles/s43018-020-00139-8 1/N
The idea for this originated now nearly 4 years ago, with an observation @GeneCollector's observation that TMB associates with response to checkpoint inhibitors and the observations by @ldiaz1 and others that DNA repair defects, like mismatch repair, can increase TMB 2/N
BRCA1 and BRCA2 are members of the homologous recombination (HR) DNA double-strand break repair pathway, one of the most commonly affected DNA repair pathways in cancer. We had previously shown cause a mutational signature pan-cancer and defects modestly increase TMB. 3/N
Some preliminary observations by other groups had suggested BRCA2 may associate with response to ICI (Hugo, et. al., Cell, 2016) and that knocking out BRCA2 can lead to inflammatory signaling (Heijink, Nat Comm, 2019). 4/N
We initially hypothesized that alterations in HR would improve response to checkpoint inhibitors (ICI) in humans. Analyzing MSK-IMPACT data of patients who received ICI for mutations in pathways, identified that patients with HR mutations had improved OS after ICI. 5/N
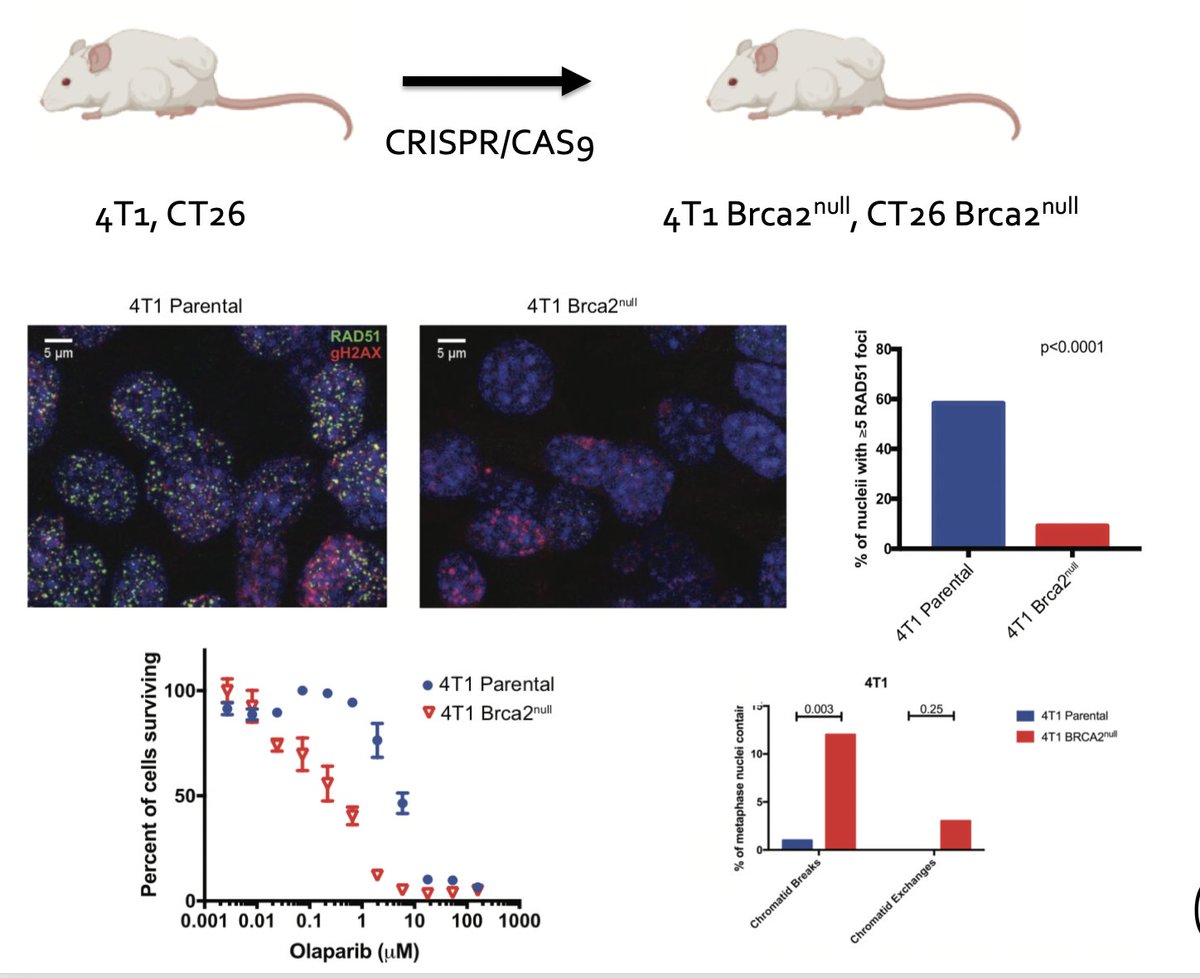
@rsamstein wanted to functionally test this & developed syngeneic murine models of HR deficiency. He developed BRCA2-null models in 4T1 and CT26 that had canonical features of HRD, including decreased RAD51 foci after IR, increased chromatic breaks, and sensitivity to PARP(i) 6/N
In mice, these models demonstrated elevation in both CD8 and CD4 T-cell infiltrate. Both 4T1 and CT26 BRCA2-null tumors were significantly more sensitive to ICI than parental controls (7/N)
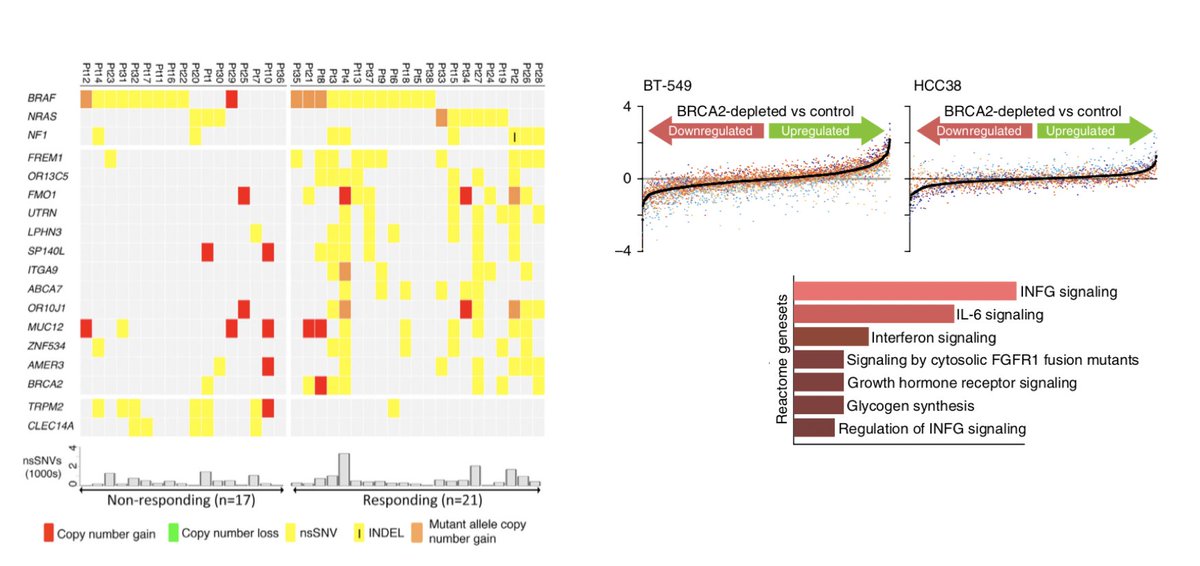
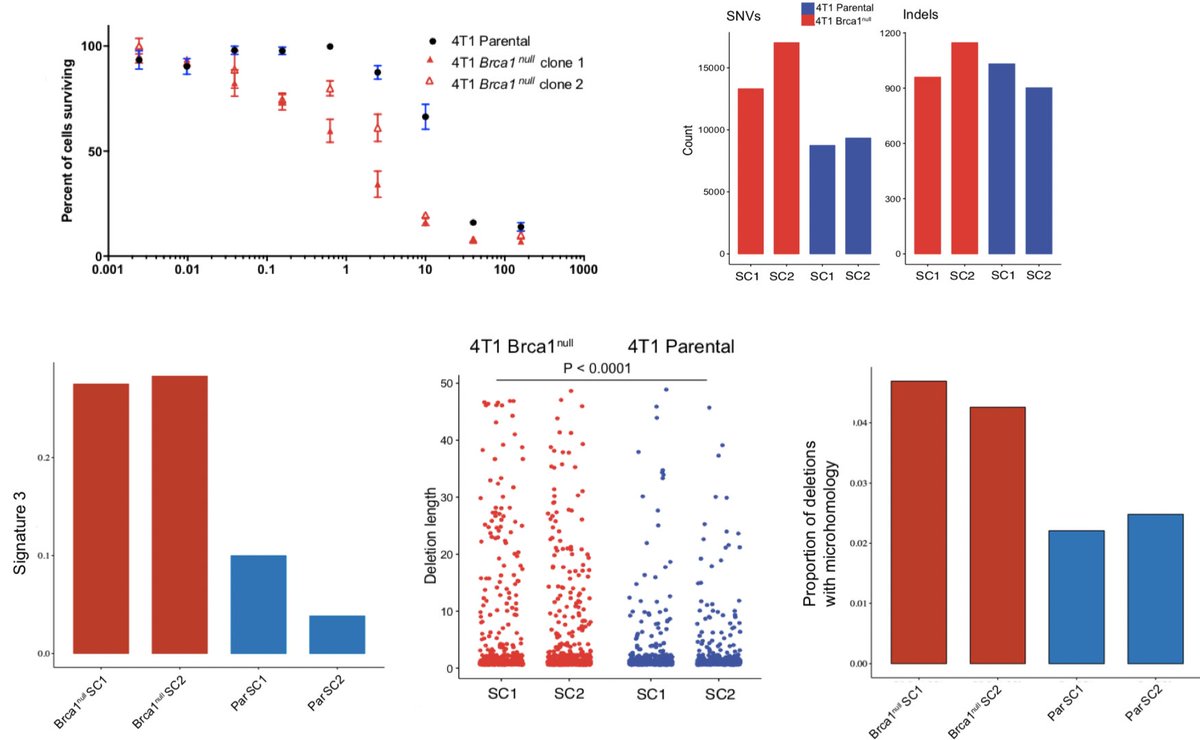
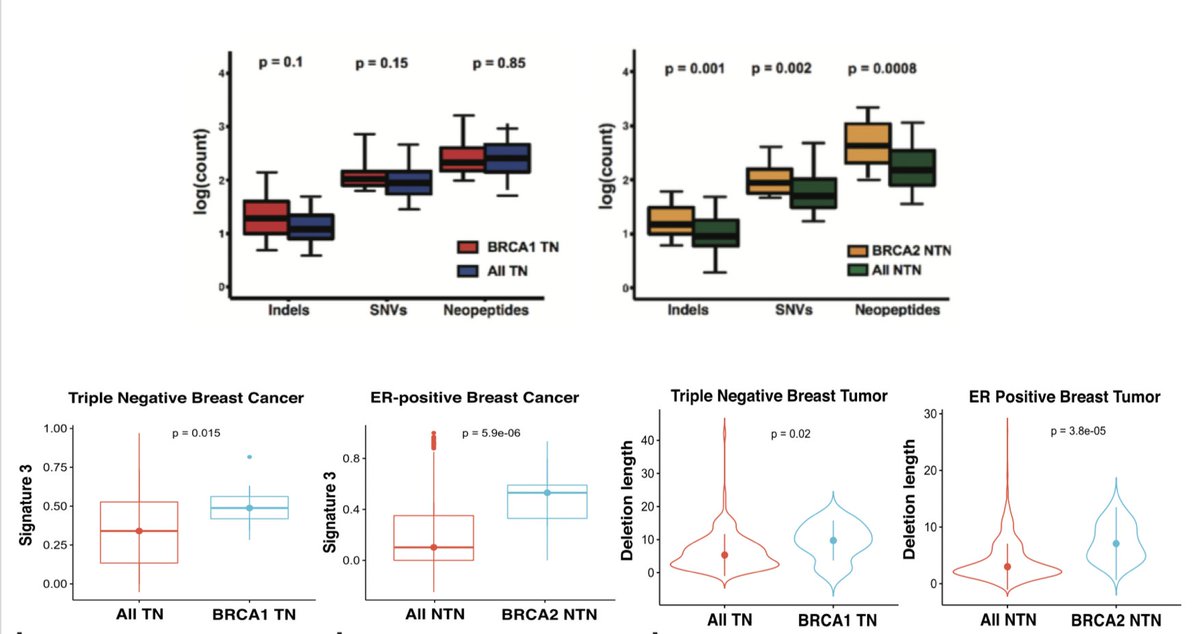
We performed WGS on these models & identified characteristic mutational signatures of HRD described by @SerenaNikZainal & others. They had an increased TMB, in indels, an increase in signature 3 related mutagenesis, & an increase in indels with micro-homology. (8/N)
@rsamstein subsequently developed a 4T1 BRCA1-null model, which again demonstrated canonical features of HRD, parp(i) sensitivity, increase in TMB, signature 3 mutagenesis, and indels with microhomology. Interestingly, numerically, indels were only slightly higher. (9/N)
To our surprise, the 4T1 BRCA1-null model was NOT sensitive to ICI. We first were not sure what to make of this, but contemporaneous work from Geoffrey Lindeman (Nolan, et. al., Sci Trans Med), group showed similar results in a GEMM model of BRCA1 deficiency. (10/N)
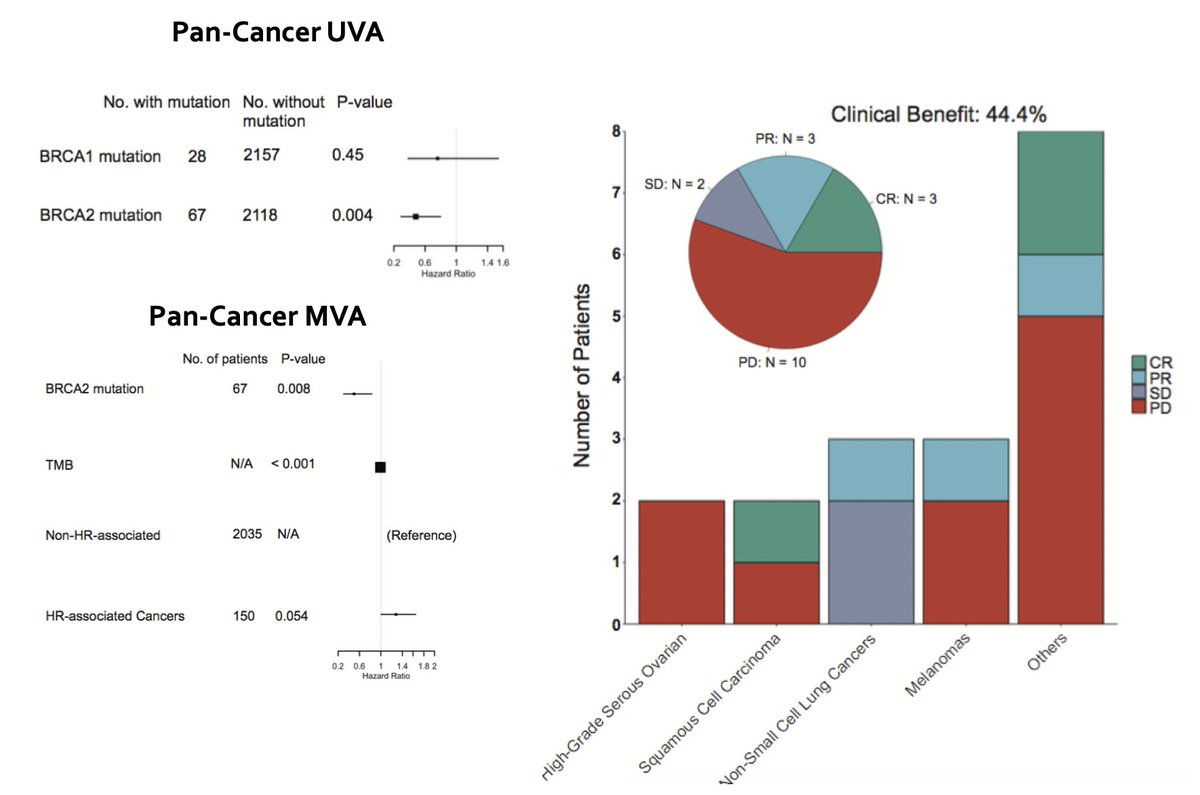
We went back to our human data & performed a pan-cancer analysis that indeed found BRCA2 and not BRCA1 was associated with OS after ICI. This remained true after MVA correcting for TMB. Together, the findings suggest BRCA1 and BRCA2 have distinct effects on immunity. (11/N)
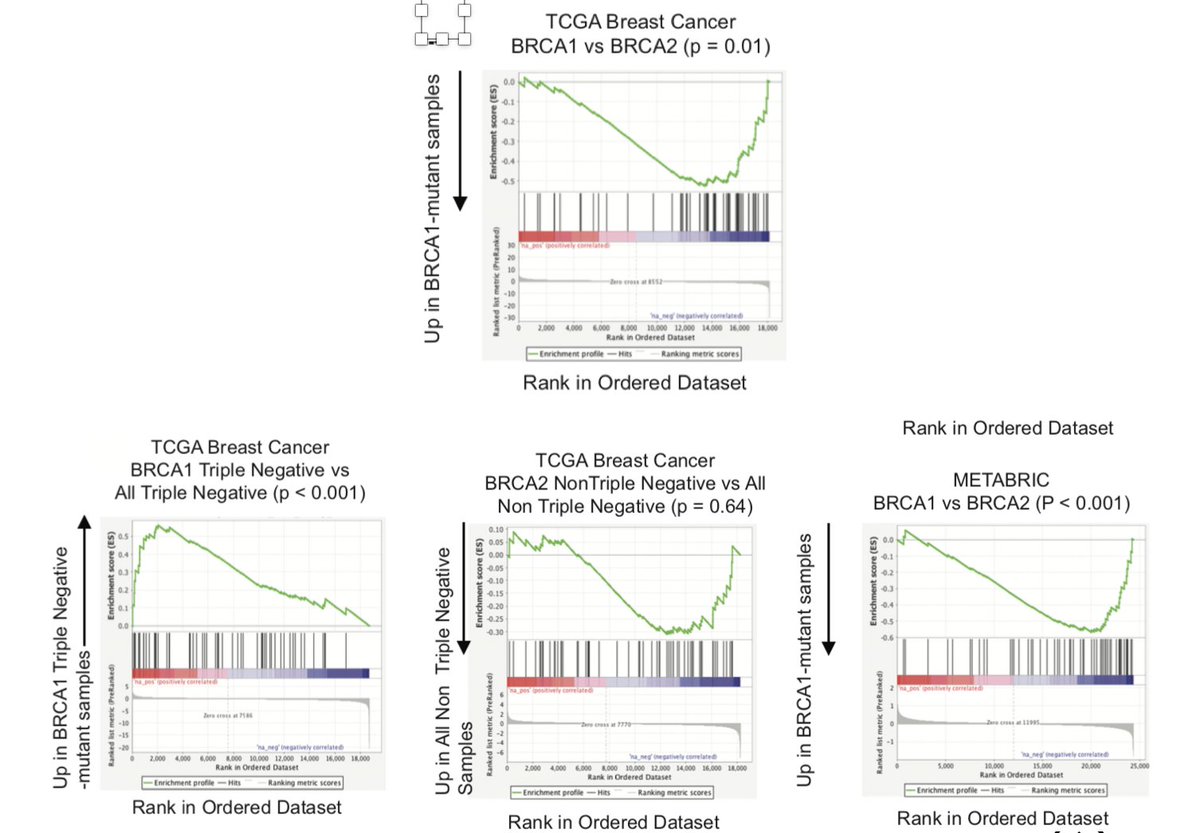
We next wanted to examine directly influences on the genome of BRCA1 and BRCA2 aberrations in breast cancer, compared to histologic controls. With help of Jorge Reis-Filho, we noted BRCA2 tumor had a more profound influence on the genome compared to BRCA1 (12/N)
In combination with the mutational process findings from our model systems, this suggests that differences in mutational process between BRCA1 and BRCA2 likely contribute to differences in anti-tumor immunity (13/N)
We next turned our attention to possible differences in intra-cellular immune signaling Performing RNA-seq in culture on our BRCA1 and BRCA2 cell lines, @chirag_msk, identified an immunoregulatory set of genes that was enriched in the BRCA1 model. (14/N)
Surprisingly, we found this same gene set was also enriched in human breast cancers from TCGA, comparing BRCA1 and BRCA2 directly. Since these occur in distinct phenotypes (ER+, triple negative), we found BRCA1 tumors were enriched vs. TN, but not BRCA2 vs. ER+. (15/N)
This finding also reproduced in breast cancers from METABRIC, and suggested that BRCA1 alteration may induce an intrinsic immunosuppressive program (16/N)
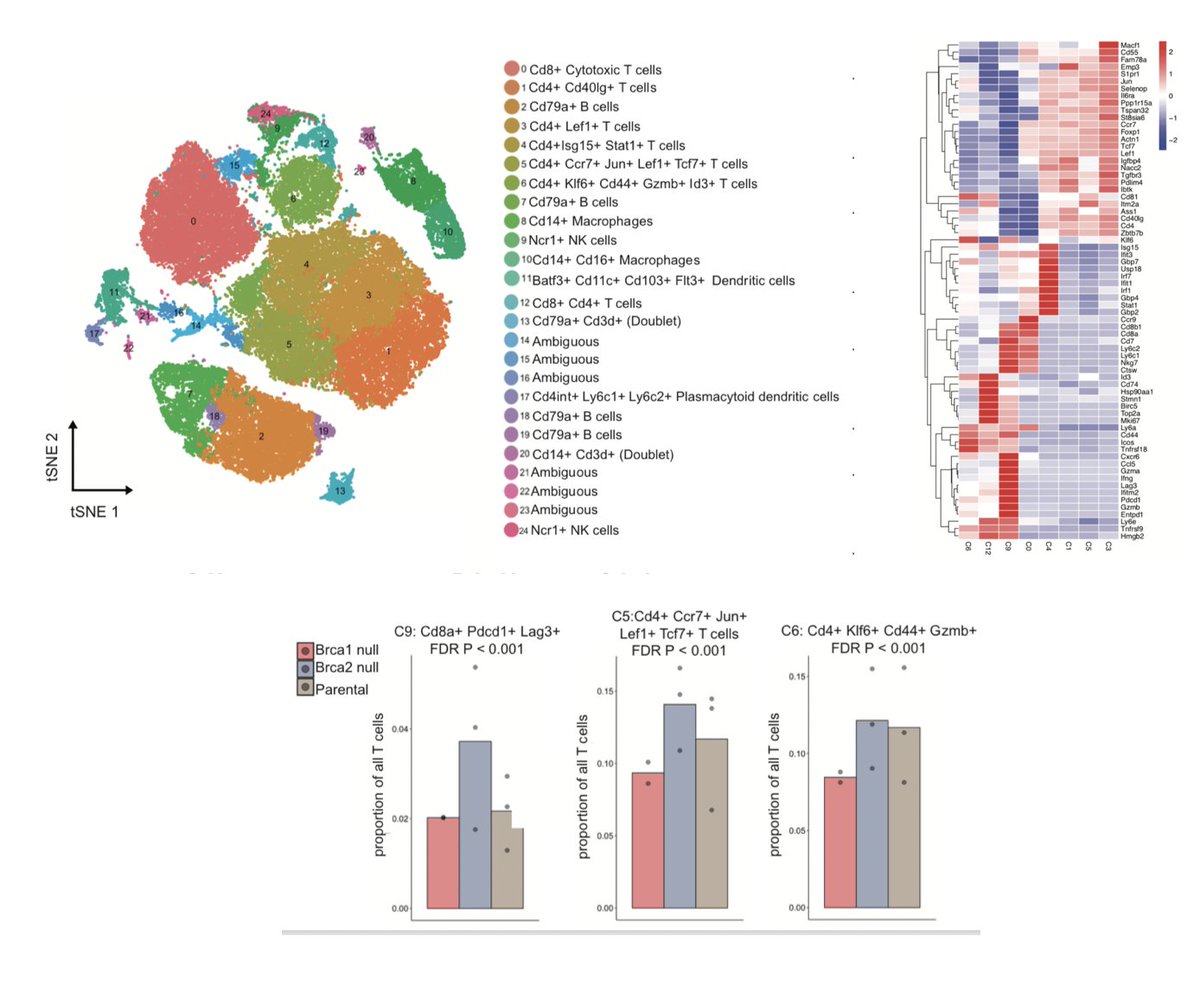
Lastly, @chirag_msk, xiaoxiao & @rsamstein used single-cell RNAseq to identify differences in the TME of our murine models. They found an enrichment for an activated/exh. CD8 pop, an NK cell pop, and a stem-like CD4 cell population in the BRCA2 tumors. (17/N)
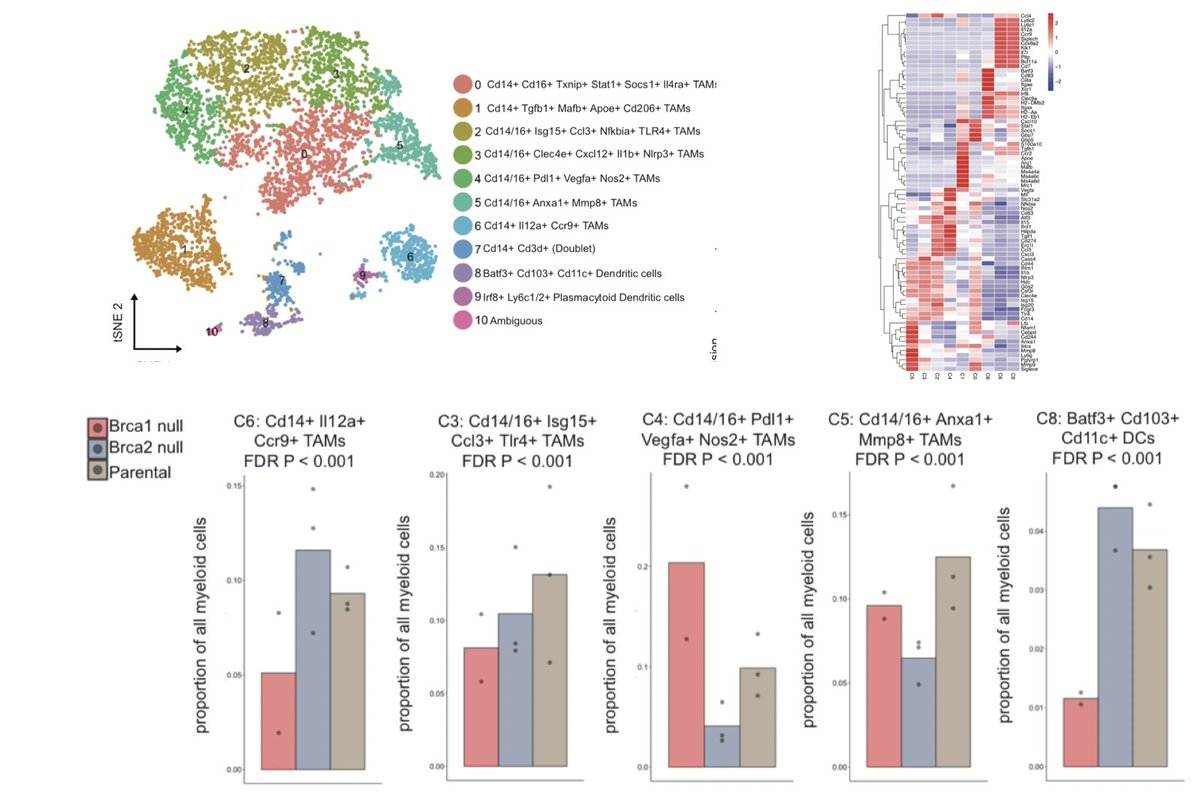
An evaluation of the myeloid compartment, noted significant differences in TAM subsets between BRCA1 and BRCA2 and a depletion of DCs in the BRCA1 model. (18/N)
Im sum, (i) BRCA1 and BRCA2 tumors have distinct effects on immunity in mice and people. (ii) This is partially mediated by distinct mutational landscape & an immunoregulatory program upreg. in BRCA1 (iii) scRNA-seq found diff in adap & innate immunity in TME (19/N)
Of note, the recent publication of CheckMate-650, run by Padmanee Sharma, also suggested improved immunity in BRCA2 tumors (20/N)

 Read on Twitter
Read on Twitter