It's been a day. Time to wrap it up with a tweetorial thread (my first) about my latest paper out in PlosOne today. Great timing considering I'm scheduled to give a talk on this tomorrow for #CATMo2020.
Let me present: 'extreme' adaptive therapy.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0243386
1/18
Let me present: 'extreme' adaptive therapy.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0243386
1/18
I have extensively analyzed (read: beaten the dead horse) the model that informed the adaptive therapy clinical trial in mCRPC, but with the apparent success of the trial, it's worth doing a bit more.
https://www.nature.com/articles/s41467-017-01968-5
2/18
https://www.nature.com/articles/s41467-017-01968-5
2/18
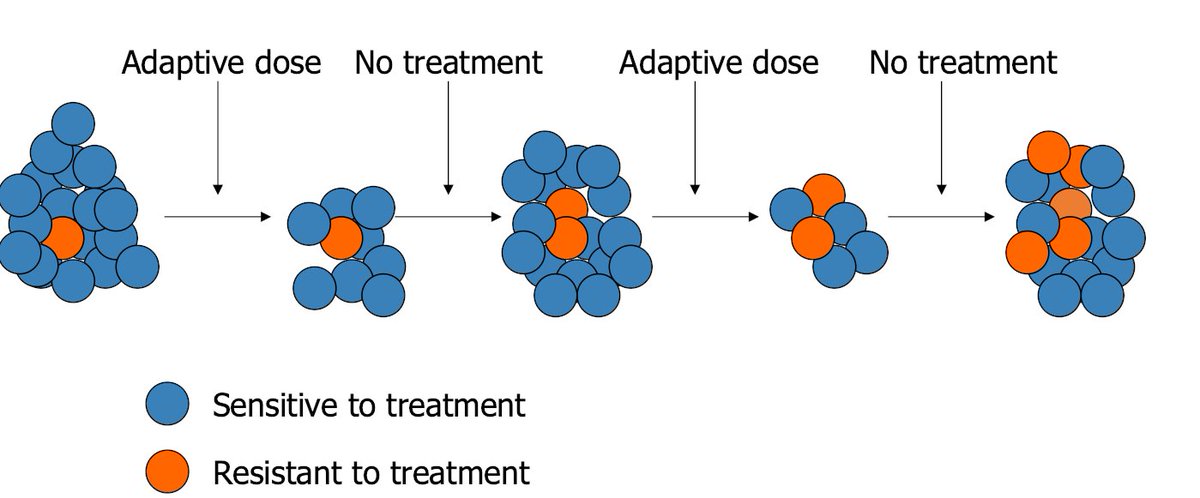
For those new to adaptive therapy: We have a drug that kills cells sensitive to it and we use it at constant high doses to attempt a cure. In metastatic disease we never cure. Why? You know how Lysol kills only 99.99% of germs? Yeah... Same idea.
3/18
3/18
The 0.01% of cancer cells left eat our drug for breakfast, grow like mad, and we don't have anything to stop them. This is a tale as old as cancer treatment itself. So what can we do? Well, we know the drug works on a lot cells, so why did we get rid of them all so fast?
4/18
4/18
The adaptive therapy trial took patients with mCRPC and treated them with Abiraterone until the tumor burden cut in half. Then we stopped treatment and let the tumor grow back. Scary thought. BUT. When the tumor gets as big as it was when you came in we hit it again.
5/18
5/18
Come to find out the majority of patients cycle multiple times, increasing the length of time that the single drug Abiraterone remains effective. Magic! But, patients still progress, resistant cells eventually win out. So while we're in better shape, it's not perfect.
6/18
6/18
Why 50% drop you ask? Well, the model said it would work pretty well and the physician agreed to do it. Other than that it's a bit arbitrary. Can we do better? You bet we can. Dropping the PSA only 40% works better by leaving around more sensitive cells. 25% yet better!
7/18
7/18
So... Taking this to the extreme why not drop the PSA by 0%? What!? Keep the PSA constant you say? Yes. Why not? IF (and this is the big if) the patient has good quality of life with their current tumor burden, why mess with it?
8/18
8/18
Keeping a constant PSA means stabilizing the tumor at a constant volume. Good news. Evolutionary game theory comes with the notion of stable equilibria. In this case the equilibria are compositions of tumors that can remain constant indefinitely.
9/18
9/18
Ooooo. I like the word indefinitely when it comes to peoples lives. So, if a stable equilibria exists, how can we apply Abiraterone to arrive at a stable equilibria when starting from any initial tumor composition and stay there for ever and ever and everrrrr?
10/18
10/18
I wouldn't be here if there wasn't a fun answer. With some fancy numerical optimal control shenanigans, the answer is.... drum roll please... dose titration! What? Dose titration? Like... slowly ramping up a drug dose until the desired effect is achieved? You bet.
11/18
11/18
Dose titration or 'ramp up' does three things. 1) Delays treatment as long as possible to ensure a large population of sensitive cells, 2) Uses the minimum effective dose to maintain quality of life, and 3) Uses patient specific feedback to drive treatment in real time.
12/18
12/18
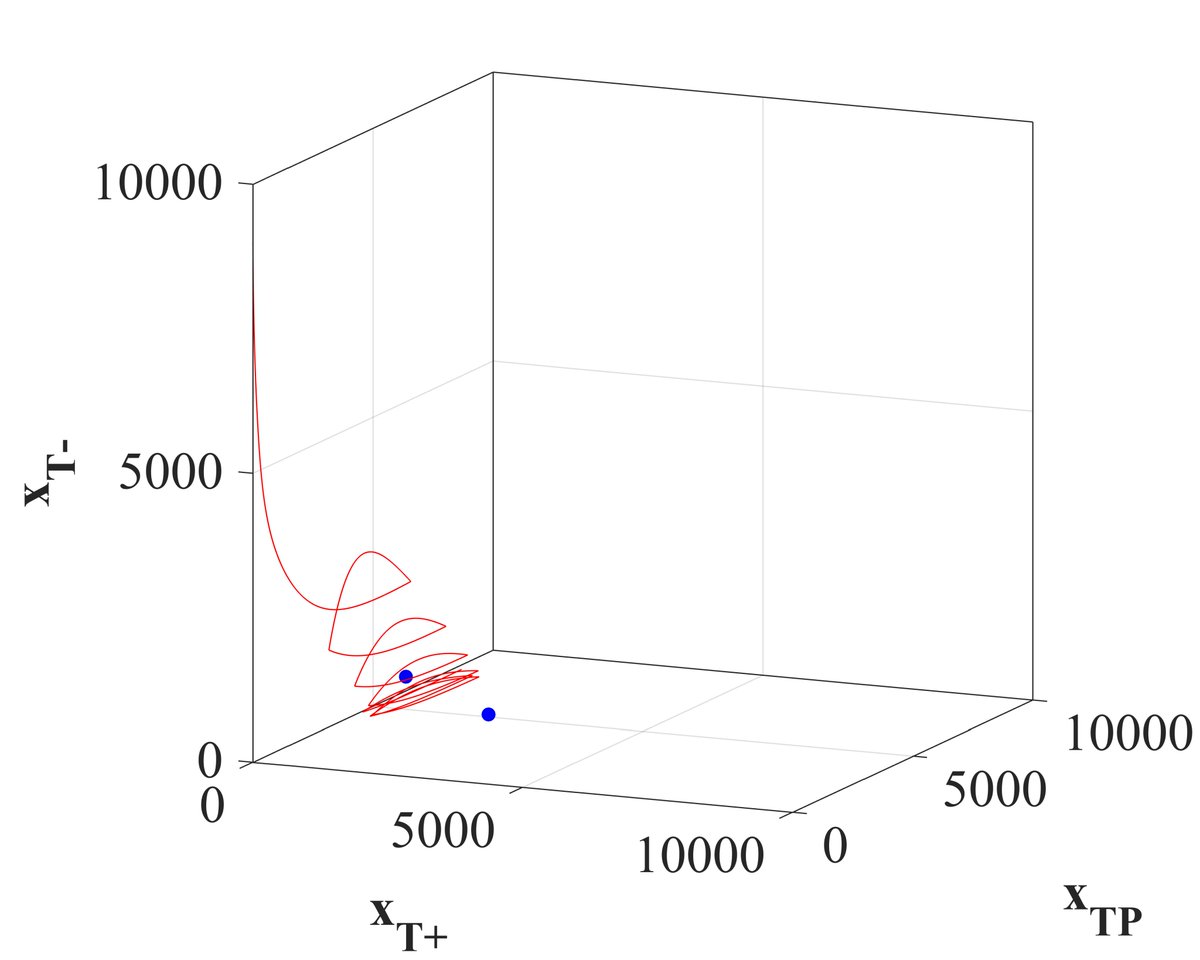
Below, x and y-axis are treatable cell types. z-axis is the resistant cell type. Traversing up is bad. Current standard of care and even 50% adaptive therapy drive tumor compositions to full resistance, missing any stable equilibria, shown as the two little blue dots.
13/18
13/18
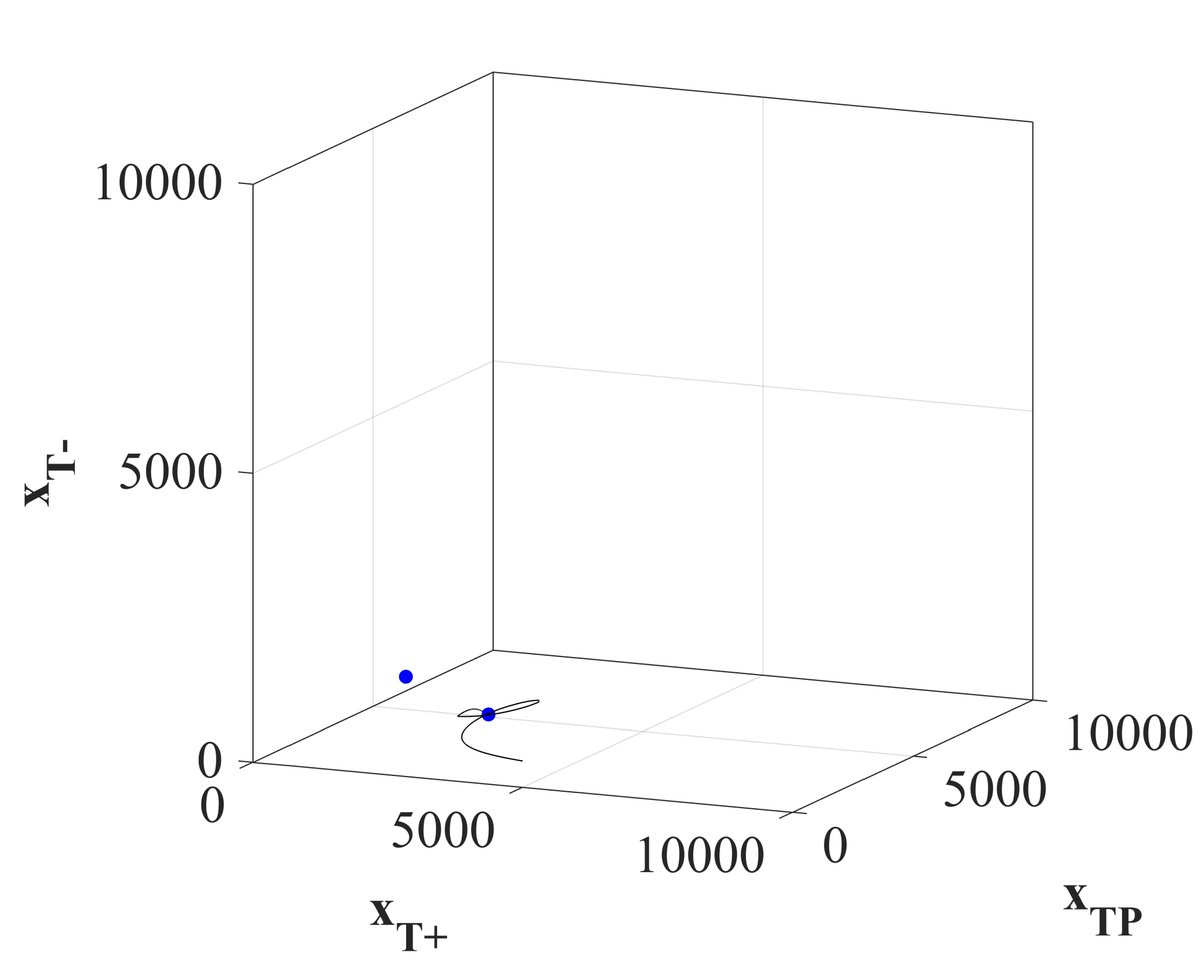
Lo and behold the beauty of titration! The tumor composition is directed towards a stable equilibria and can be maintained at that stable equilibria indefinitely.
14/18
14/18
I would break Twitter if I tried to list all of the caveats that go along with dose titration for treatment of mCRPC. Sometimes titration vastly over-treats, sometimes it vastly under-treats. Go to the paper if you want to hear the bad stuff. I'm staying positive here.
15/18
15/18
There are hints of success of titration but no explanation of why. Titration of axitinib resulted in a greater proportion of patients with metastatic renal cell carcinoma achieving an objective response. Did they keep around more sensitive cells?
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4120767/
16/18
https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4120767/
16/18
Incredibly, titration of regorafenib in patients with metastatic colorectal cancer actually increased median overall survival from 5.9 months (initiating treatment at standard dose) to 9.0 months. Were they close to achieving equilibrium?
https://www.annalsofoncology.org/article/S0923-7534(19)33700-7/fulltext
17/18
https://www.annalsofoncology.org/article/S0923-7534(19)33700-7/fulltext
17/18
With titration used in other clinical spaces, it is exciting to think of it in treatment of metastatic disease. When we reject “cure” we must replace our definition of success. Success here is dying of something else while happening to have some tumor in you.
Fin!
18/18
Fin!
18/18

 Read on Twitter
Read on Twitter