PACLITAXEL TWEETORIAL
1/Brief tweetorial to provide context of the importance of today's NEJM paper showing no mortality difference at 1-4 years between patients with peripheral artery disease treated with paclitaxel-coated (PCD) & non-coated devices.
https://www.nejm.org/doi/full/10.1056/NEJMoa2005206?query=featured_home
1/Brief tweetorial to provide context of the importance of today's NEJM paper showing no mortality difference at 1-4 years between patients with peripheral artery disease treated with paclitaxel-coated (PCD) & non-coated devices.
https://www.nejm.org/doi/full/10.1056/NEJMoa2005206?query=featured_home
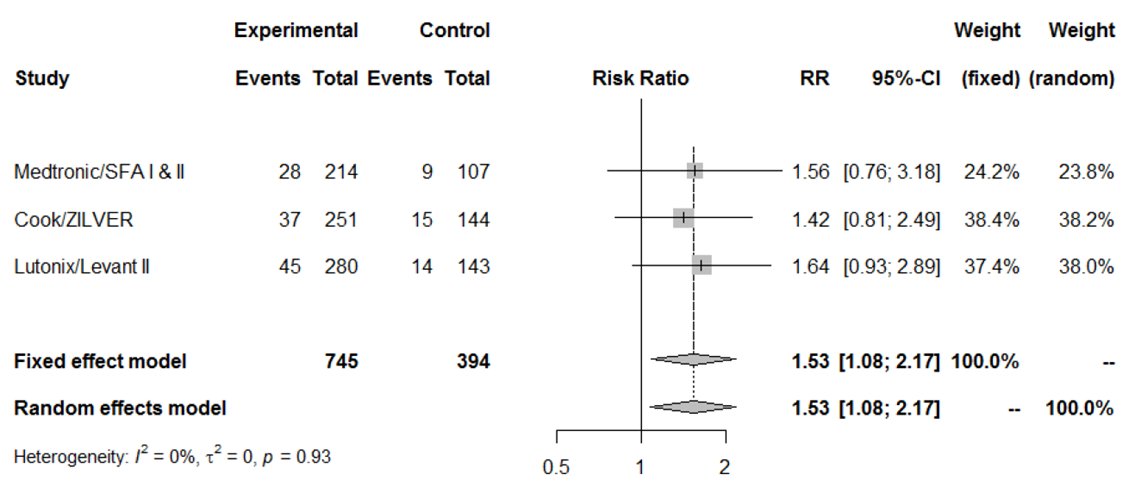
2/Exactly 2 years ago a meta-analysis of RCTs was published in JAHA that found PCDs (balloons & stents) were associated w/a 3.4% increased risk of all-cause death in 12 trials at 2 yrs & a 6.5% increased risk in 3 trials at 4-5 yrs versus non-PCDs.
https://www.ahajournals.org/doi/full/10.1161/JAHA.118.011245
https://www.ahajournals.org/doi/full/10.1161/JAHA.118.011245
3/The study had flaws. There was no mechanism of harm. Causes of death didnt differ between groups. There was an unclear relationship between more PCDs & death. Stents & balloons were analyzed together. Pts differed in characteristics. There was no censoring for lost to follow-up
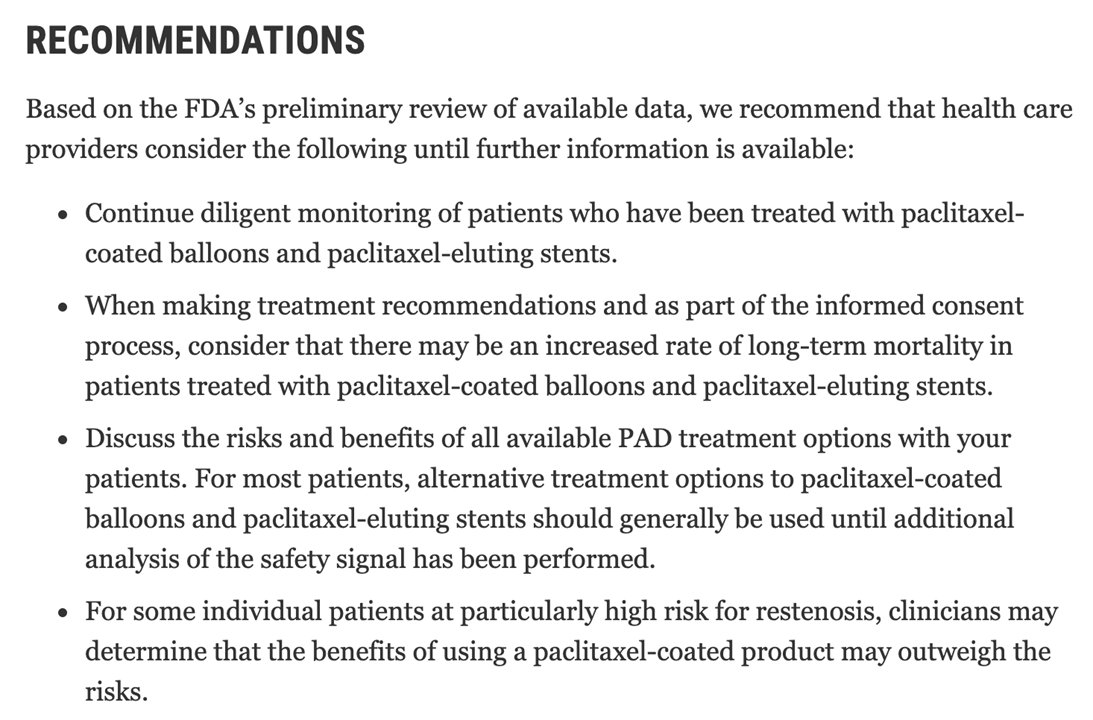
4/Despite this, the study had an immediate impact. Randomized trials (including the NEJM SWEDEPAD Trial published today) were halted and the FDA began an internal review of the data. Devices were pulled off the shelves at hospitals across the world.
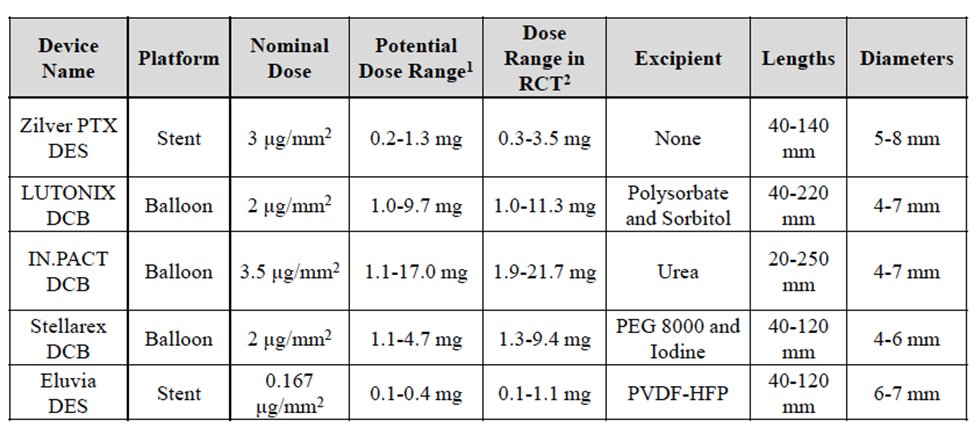
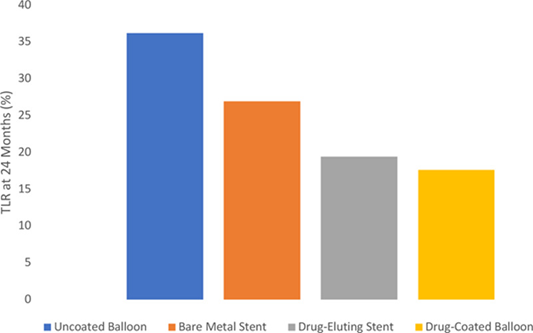
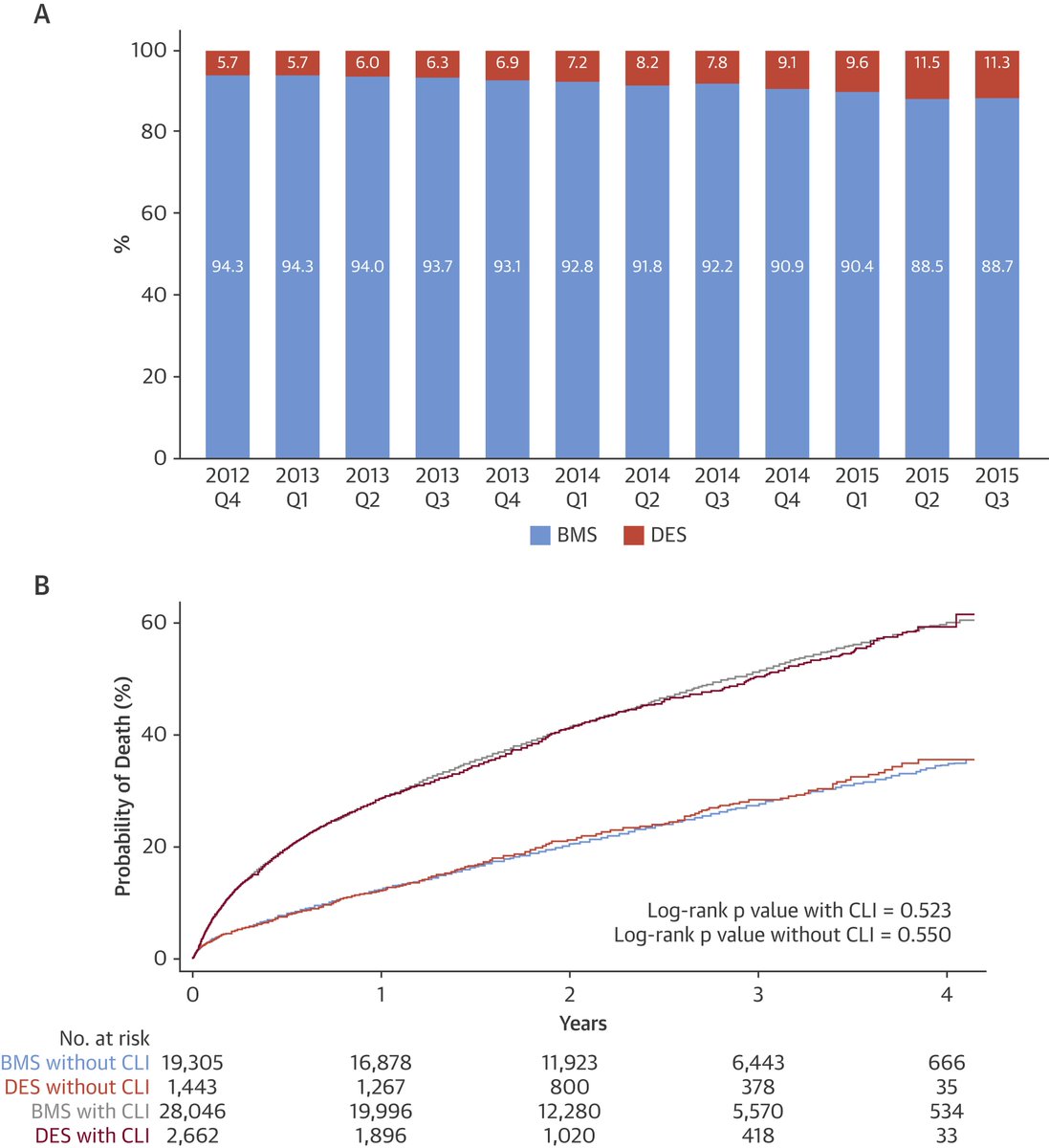
5/This had a major impact on pt care. Paclitaxel-coated stents were approved in 2012 & paclitaxel balloons in 2015. These devices addressed a major issue with short-term restenosis after intervention. These devices quickly replaced uncoated therapy & were endorsed by societies.
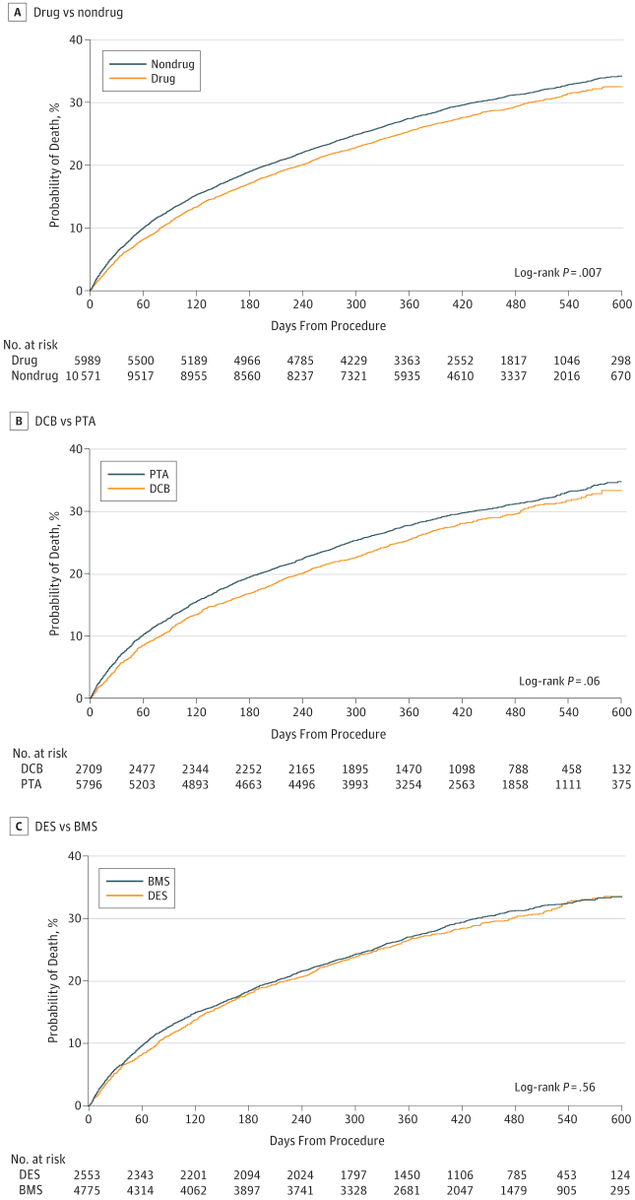
6/We @SmithBIDMC were concerned about the issues with the meta-analysis. As PAD rates increase w/age, we turned to CMS data to reproduce these findings. We couldn't. Our 1st analysis in @JAMACardio involving ~16000 patients found no harm through 600 days.
https://jamanetwork.com/journals/jamacardiology/fullarticle/2725047
https://jamanetwork.com/journals/jamacardiology/fullarticle/2725047
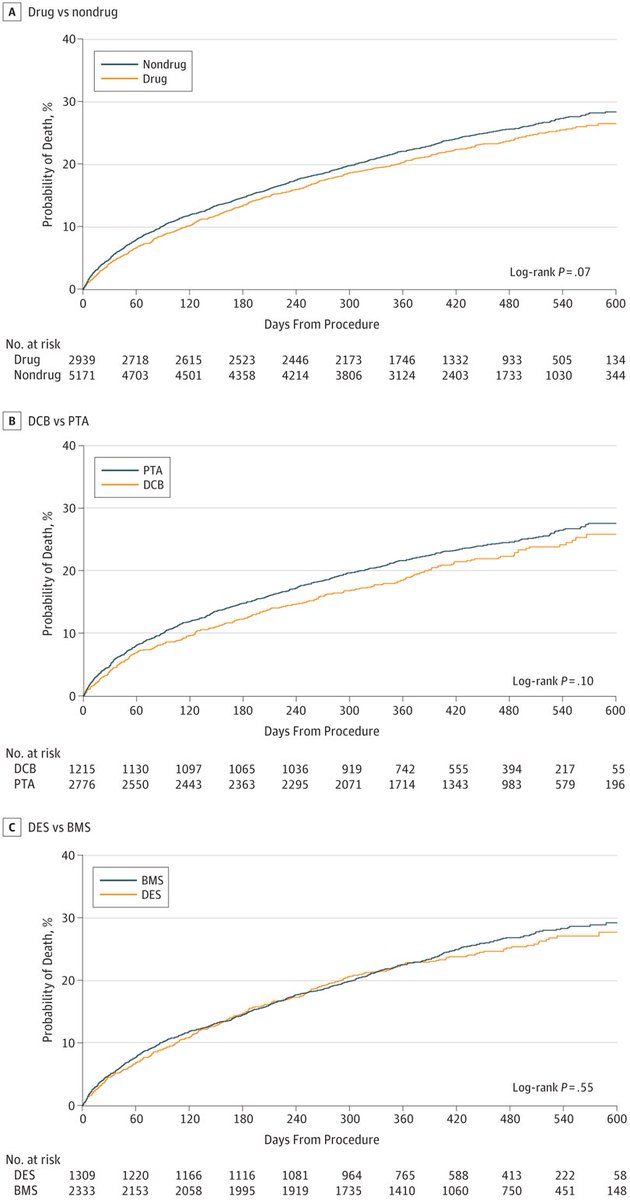
7/We followed this up with a separate analysis in @JACCJournals using more historical CMS data and focusing on paclitaxel-coated stents vs bare metal stents. This time we had ~50,000 patients and >4 years of follow-up. Again, no evidence of harm.
https://www.jacc.org/doi/full/10.1016/j.jacc.2019.02.020
https://www.jacc.org/doi/full/10.1016/j.jacc.2019.02.020
8/Despite this, the FDA were able to reproduce the findings of the meta-analysis in their internal data. They issued a warning to health-care providers to restrict use. A safety panel was planned for June 2019. @SmithBIDMC We were asked by the FDA to expand on our prior CMS work.
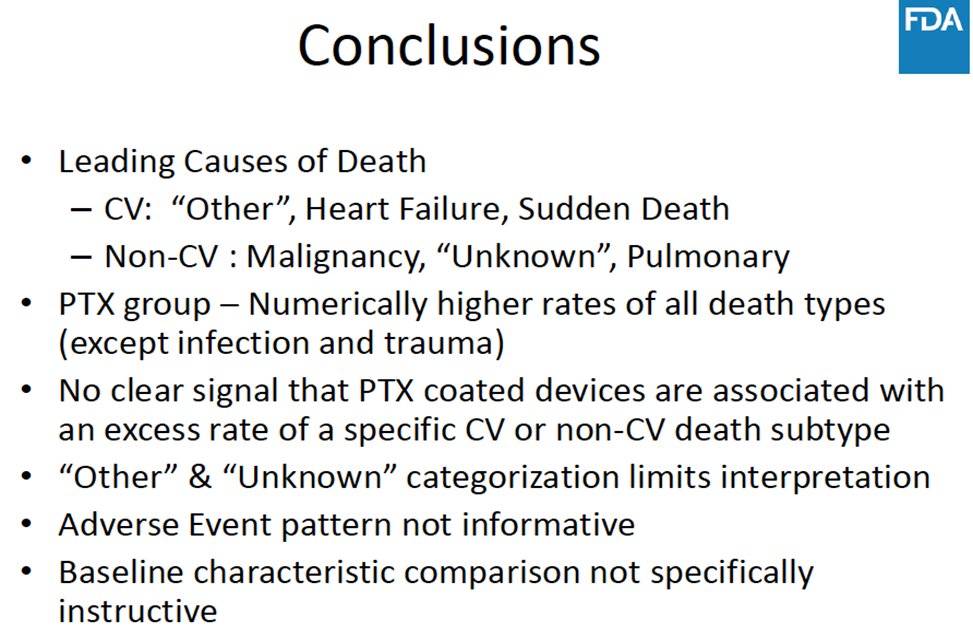
9/At the panel, the FDA presented their internal data. Although a signal of harm persisted, they could not find a clear mechanism or cause of death. Device manufacturers presented individual trial data that also failed to reproduce the harm seen in the composite meta-analysis.
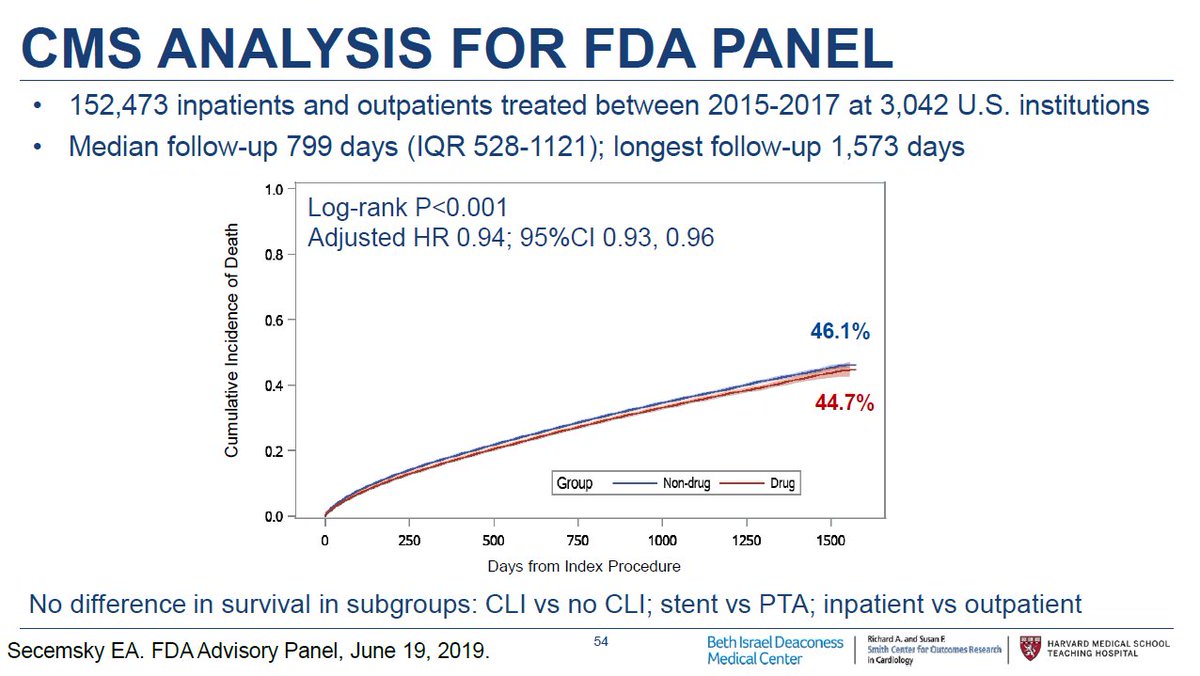
10/ @SmithBIDMC presented CMS data involving >150,000 pts treated both at inpatient and outpatient centers. Mortality follow-up was available through April 2019 & surpassed 4 years. We looked at key subgroups & presented an instrumental variable analysis. Again no signal of harm.
11/We also worked with Optum data to explore the signal among patients with Medicare Advantage. Among >16000 patients, we again found no evidence of harm.
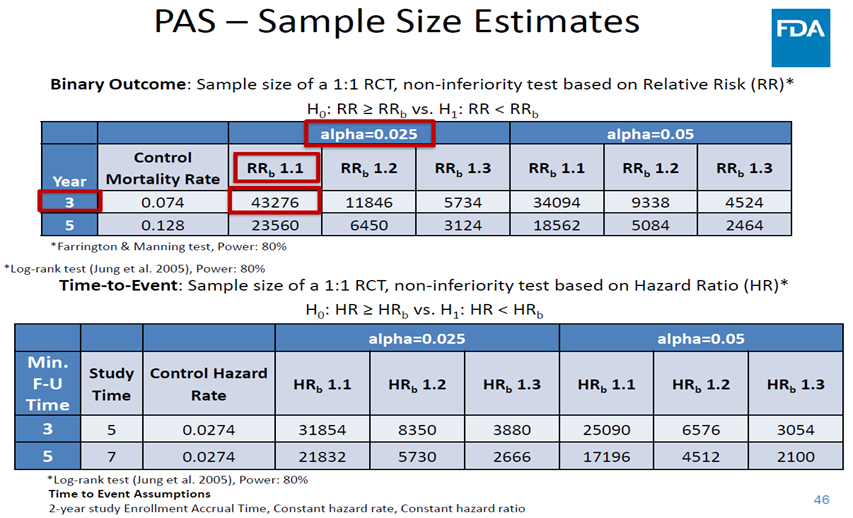
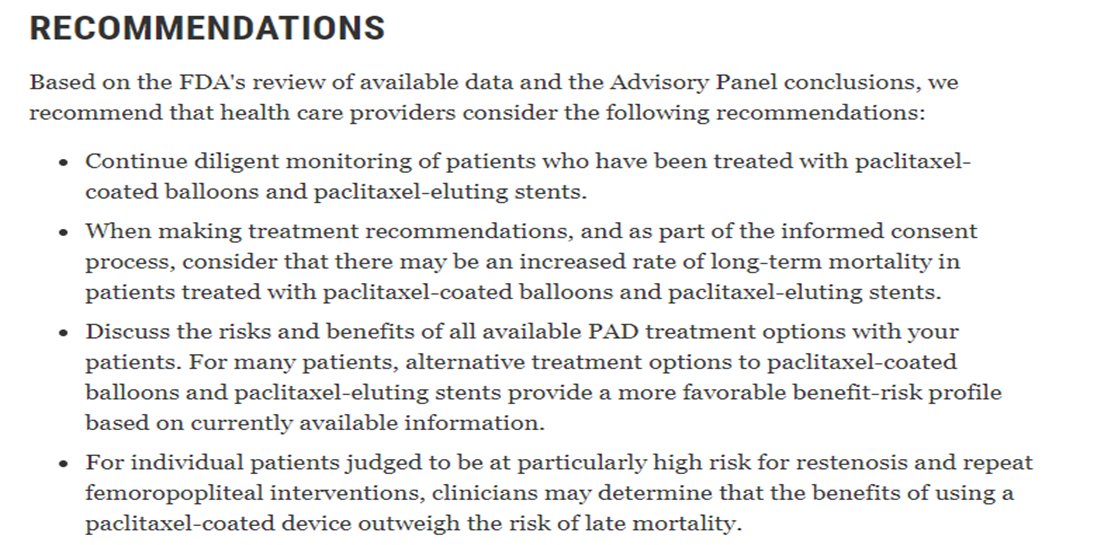
12/The FDA concluded the panel by allowing devices to remain on the market. They issued a revised letter to providers again restricting use & device labels were updated to include this signal of harm. The FDA noted a RCT powered for death would be infeasible & requested more data
13/ @SmithBIDMC was engaged to design a study using CMS data to provide a longitudinal mechanism to evaluate this harm signal. This prespecified analysis designed w/the FDA called SAFE-PAD will continue until '23 when all pts reach a >5 years of follow-up.
https://clinicaltrials.gov/ct2/show/NCT04496544
https://clinicaltrials.gov/ct2/show/NCT04496544
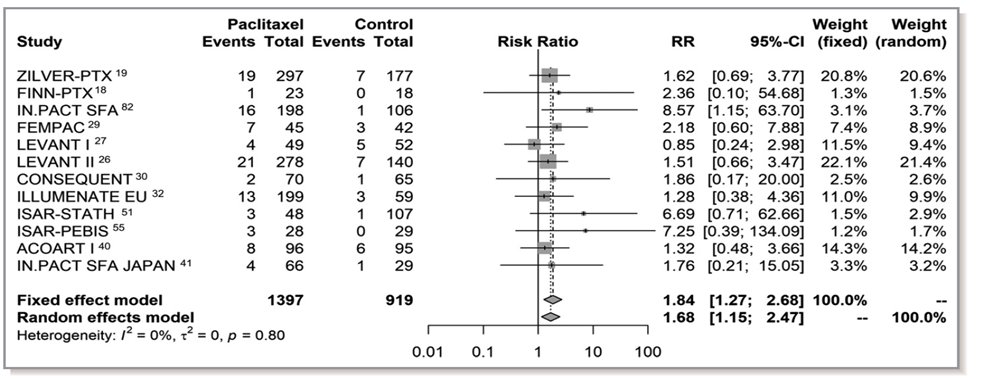
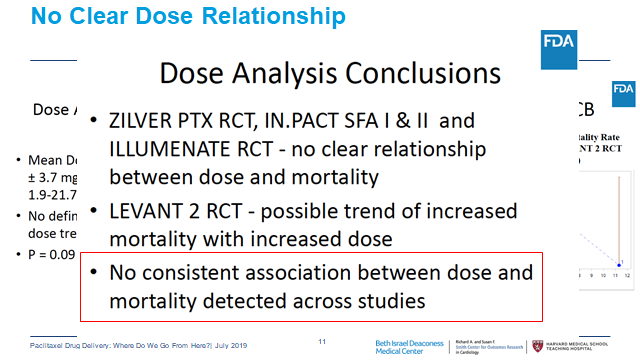
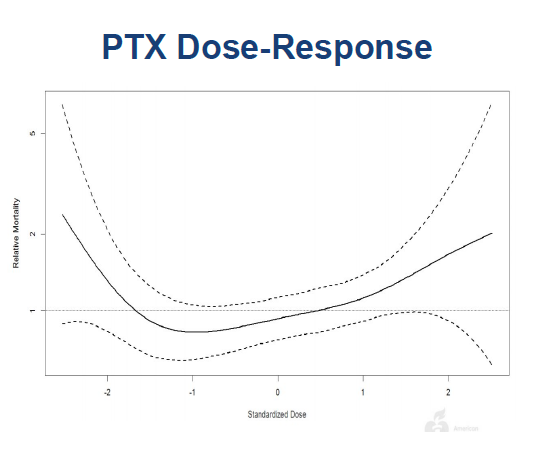
14/In the meantime critical data have been published. A patient-level meta-analysis of RCTs by @VIVAPhysicians showed the harm signal attenuated as pts lost to follow-up were included. This makes no sense if missingness was random. Also again showed no dose-mortality relationship
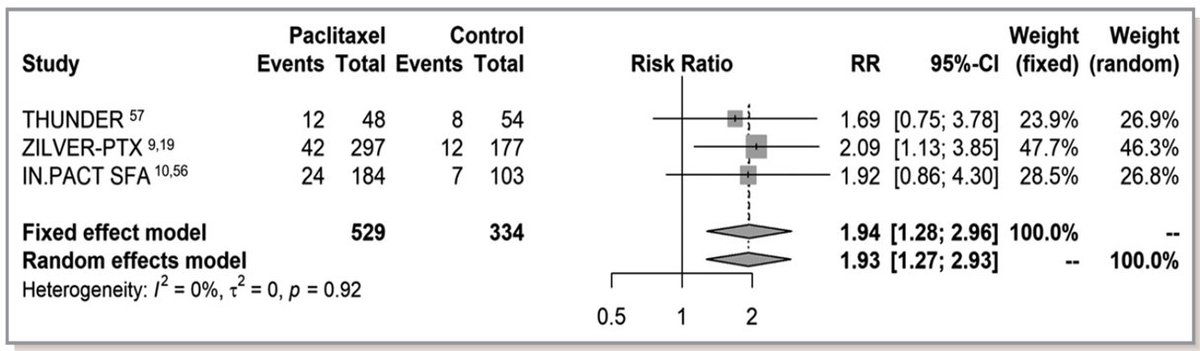
15/A recent sub-analysis of the VOYAGER PAD RCT found no long-term harm between PCDs and non-PCDs. And then today, we have interim results from likely the only RCT that randomized to PCDs vs no PCDs and again no evidence of harm.
16/My take: the intial RCTs were never designed to evaluate long-term mortality & pts who had mortality data available were much different than those who did not. Also these meta-analyses involved <1500 pts with very different risk profiles and treated with different devices.
Fin/Patient care has suffered without the availability of PCDs. I hope we have a resolution soon. Also, real-world data played a critical role here. This was one of the intents of the 21st Century Cures Act & is part of our mission of @SmithBIDMC. Thanks to @rwyeh @Changyushen312

 Read on Twitter
Read on Twitter