Q: Why do we use a stethoscope?
A: For many reasons, and here’s one of them that I will argue is undervalued. And is still at the heart (hint hint) of some ongoing research…
A: For many reasons, and here’s one of them that I will argue is undervalued. And is still at the heart (hint hint) of some ongoing research…
First described by Potain in 1880, the gallop rhythm refers to the presence and cadence of abnormal diastolic cardiac sounds, namely S3 and S4.
In 1989, Stevenson and Perloff described the previously unestablished relationship between physical exam signs, increased ventricular filling pressures, and decreased cardiac output in chronic HF patients. Stevenson et al. JAMA. 1989. https://jamanetwork.com/journals/jama/fullarticle/376284
An excellent point is made in this paper: “While the relationship between physical signs and hemodynamic profile has a firm basis for acute congestive heart failure, the chronic state is characterized by a host of compensatory mechanisms that may cause disparities, …”
“…such as the absence of rales and peripheral edema despite symptomatic elevation of ventricular filling pressures.”
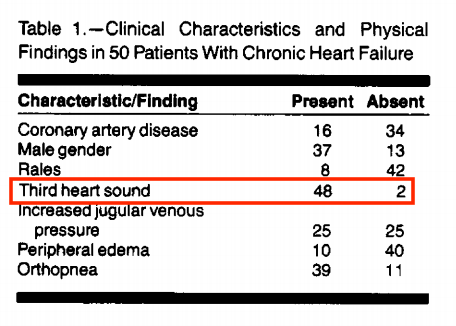
This cohort had quite the notable med regimen: digoxin (84%), furosemide (84%), vasodilators (56%), and milrinone (4%); 39/50 were being evaluated for transplant
This cohort had quite the notable med regimen: digoxin (84%), furosemide (84%), vasodilators (56%), and milrinone (4%); 39/50 were being evaluated for transplant
Interestingly, S3 was not specific for identifying high filling pressures in this cohort – possibly given that it was graded on a binary scale (presence vs. absence). Could it be more useful if somehow represented as a continuous variable??
Similarly, presence of rales was non-specific, only found in 19% of patients with PCWP > 22.
A plausible explanation for this observation in chronic (as opposed to acute HF):
A plausible explanation for this observation in chronic (as opposed to acute HF):
In chronic HF - a sudden elevation of pulmonary venous pressure causes rales due to extravasation of fluid into the alveoli, but chronic exudation of fluid is associated with an increase in lymphatic drainage so that the alveoli remain relatively dry and rales are absent.
Last, elevated JVP correlated tightly with other physical signs of congestion but was not particularly specific for detection of elevated filling pressures.
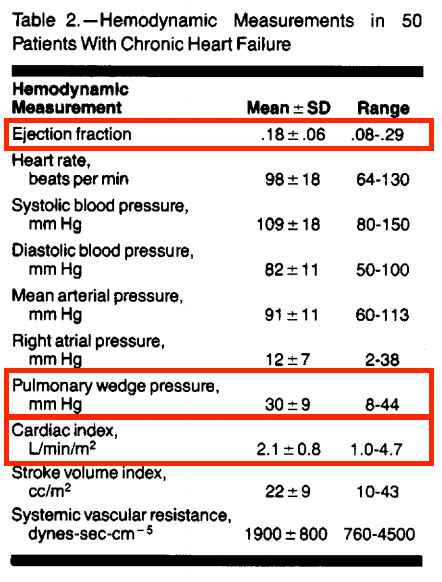
Taken together, this study suggested that marked to severe elevations of left ventricular filling pressure frequently are undetected and recommend more judicious use of invasive hemodynamic monitoring in this population.
Over a decade later, the prognostic value of the third heart sound and elevated JVP was further explored by Drazner et al. (NEJM, 2001), in a retrospective analysis of 2569 HF patients enrolled in the SOLVD trial (enalapril vs placebo for HFrEF). https://www.nejm.org/doi/full/10.1056/nejmoa010641
On univariate analysis, patients with an S3 were at significantly higher risk of death from all causes (RR 1.35 [1.17-1.55], P<0.001) and hospitalization for heart failure (RR 1.70 [1.46-1.97], P<0.001) than those without an S3.
A strength of this study was the addition of multivariate analysis to adjust for markers of HF severity (LVEF, NYHA class, medications, serum Na, etc). Contrary to what was seen in other observational studies (such as above), S3 and elevated JVP had a strong prognostic value.
In summary, presence of S3 and elevated JVP was associated with subsequent HF hospitalization, increased risk of HF progression and all-cause mortality. An association that persisted even with adjustment for markers of disease severity.
One may ask whether there is a “better” or more objective/standardized way to assess these signs, namely S3. Marcus et al. (JAMA. 2005) aimed to test this using phonocardiographic measurements to detect abnormal LV function. https://jamanetwork.com/journals/jama/fullarticle/200866
Neither the device-measured S3 nor the S4 was a sensitive marker of left ventricular dysfunction, however, phonocardiographic S3 is specific for left ventricular dysfunction and appears to be superior to the moderate specificity of the S4.
Moving forward now – we have shown that certain parameters are good prognostic factors for HF, based on how they tie into the pathophysiology of HF itself. Can we add additional measures with high clinical relevance to create a model for prediction of impending HF exacerbation?
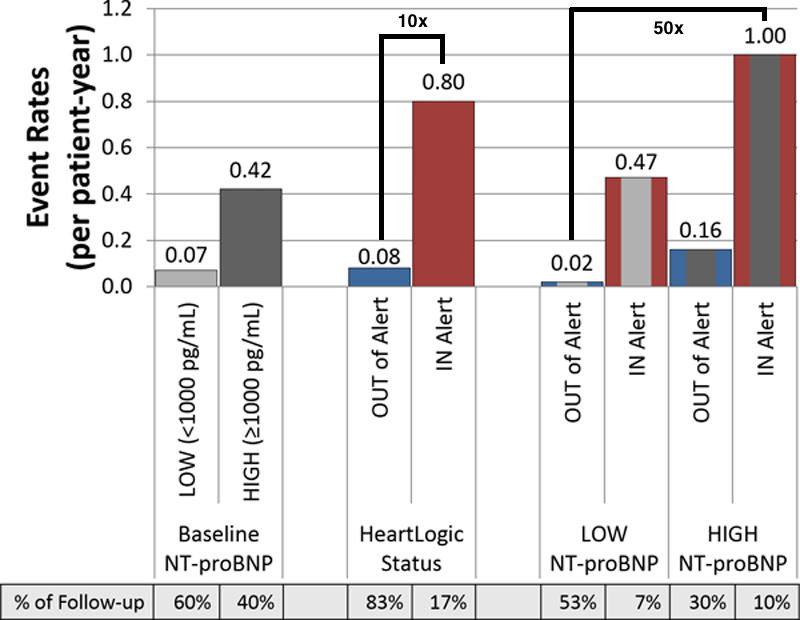
The above parameters form the HeartLogic Index used in the MultiSENSE trial - incorporated into ICD/CRT-Ds allowing for automated daily HF status eval and early awareness of impending decompensation with high sensitivity of 70% (Boehmer, JACC:HF, 2017) https://www.sciencedirect.com/science/article/pii/S2213177917300483?via%3Dihub#bib17
This algorithm was designed to detect gradual worsening of HF over days to weeks. The median early warning (alert window) was 34 days before a heart failure event, which is critical in order to provide actionable care to a patient early in the decompensation spectrum.
Further analysis of MultiSENSE (Gardner, Circ HF, 2018) showed the risk of a heart failure event while “in-alert” was 10x higher then when “out of alert” by HeartLogic Index (0.80 vs 0.08/pt-year) https://www.ahajournals.org/doi/10.1161/CIRCHEARTFAILURE.117.004669
What about biomarkers?
Substratification showed that the lowest risk group (low NT-proBNP and not in alert status) vs. the highest risk group (high NT-proBNP levels and being in alert status), had a 50-fold increased risk of an HF event (1.00/pt-year vs 0.02/pt-year).
Substratification showed that the lowest risk group (low NT-proBNP and not in alert status) vs. the highest risk group (high NT-proBNP levels and being in alert status), had a 50-fold increased risk of an HF event (1.00/pt-year vs 0.02/pt-year).
Although NT-proBNP levels had independent predictive power for HF events, it was significantly lower than that of the HeartLogic alert algorithm alone (event rates: 0.42 vs 0.80/pt-year).
Furthermore, the risk for a HF event was greater in patients with low NT-proBNP levels but in HeartLogic alert status than in subjects with high NT-proBNP levels but out of device alert status (event rates: 0.47 vs 0.16/pt-year).
If you made it this far, you must also love heart failure remote monitoring (!!)
Look how far we’ve come from simply placing a stethoscope on a patient - although this is still not obsolete, it has led to some excellent advances over the years.
Look how far we’ve come from simply placing a stethoscope on a patient - although this is still not obsolete, it has led to some excellent advances over the years.
Next, we will look at some other monitoring systems – implantable hemodynamic sensors along with wearable monitoring (a taste below). And discuss some the recent work in the field, and where things may go in the future. Until next time. https://twitter.com/AHajduczok/status/1340140815503519744?s=20
Some folks who might like this: @JeffHsuMD @FudimMarat @DrNasrien @JJheart_doc @AndrewJSauer @RyanTedfordMD @JagSinghMD @MarkDrazner @NMHheartdoc @gcfmd @HFpEF @Cardiobro @TJHeartFellows @HanCardiomd @JavedButler1 @JonathanDavisHF @MKIttlesonMD @DavidLBrownMD @CMichaelGibson etc
And our recent review article on this topic:
Ali, O., Hajduczok, A.G. & Boehmer, J.P. Remote Physiologic Monitoring for Heart Failure. Curr Cardiol Rep 22, 68 (2020). https://link.springer.com/article/10.1007/s11886-020-01309-x
Ali, O., Hajduczok, A.G. & Boehmer, J.P. Remote Physiologic Monitoring for Heart Failure. Curr Cardiol Rep 22, 68 (2020). https://link.springer.com/article/10.1007/s11886-020-01309-x

 Read on Twitter
Read on Twitter

![On univariate analysis, patients with an S3 were at significantly higher risk of death from all causes (RR 1.35 [1.17-1.55], P<0.001) and hospitalization for heart failure (RR 1.70 [1.46-1.97], P<0.001) than those without an S3. On univariate analysis, patients with an S3 were at significantly higher risk of death from all causes (RR 1.35 [1.17-1.55], P<0.001) and hospitalization for heart failure (RR 1.70 [1.46-1.97], P<0.001) than those without an S3.](https://pbs.twimg.com/media/Ep1uUDxXcAA0p2c.png)
![On univariate analysis, patients with an S3 were at significantly higher risk of death from all causes (RR 1.35 [1.17-1.55], P<0.001) and hospitalization for heart failure (RR 1.70 [1.46-1.97], P<0.001) than those without an S3. On univariate analysis, patients with an S3 were at significantly higher risk of death from all causes (RR 1.35 [1.17-1.55], P<0.001) and hospitalization for heart failure (RR 1.70 [1.46-1.97], P<0.001) than those without an S3.](https://pbs.twimg.com/media/Ep1uUFDWMAA3tUD.jpg)